About AXIM Eye

About Us
AXIM Eye is a division of AXIM Biotechnologies (OTC: AXIM), an international healthcare solutions company targeting ocular biomarkers. AXIM Eye is working with two highly specialized Point-of-Care (POC) lab testing solutions designed specifically to assist eye-care physicians in detecting and quantifying biomarkers associated with aqueous deficient dry eye disease and non-specific allergic conjunctivitis.

Our Tests
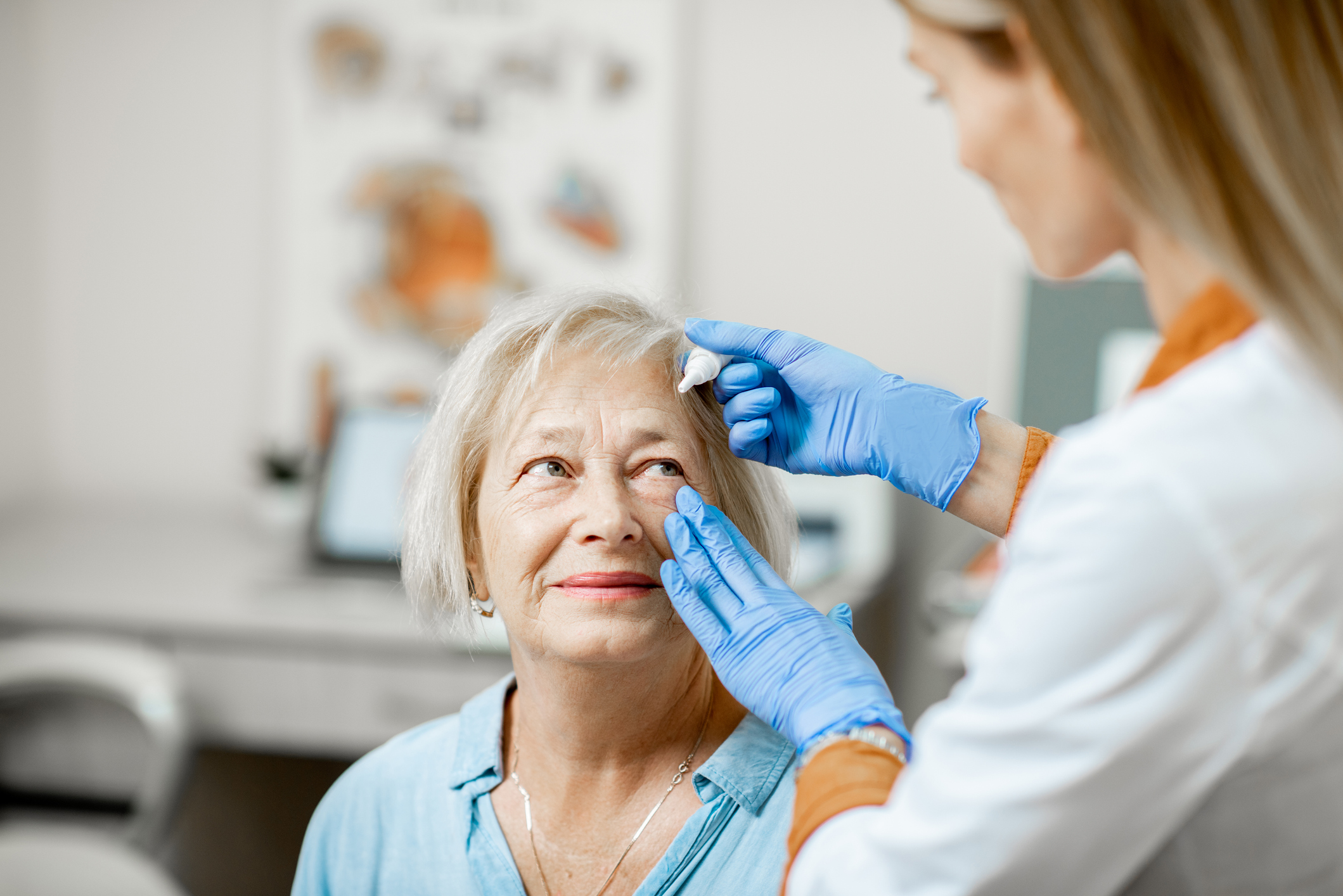
The first is a rapid Point-of-Care (8 minute) lateral flow diagnostic assay paired with a reader that tests for exact levels of Lactoferrin through the collection of 0.5 microliter in tears.
The second is designed for the measurement of Ocular Immunoglobulin E (IgE), a key biomarker primarily associated with non-specific, allergic conjunctivitis which often mimics DED and can manifest with DED.
Both tests are FDA-cleared and CMS and insurance reimbursable.
BACKGROUND
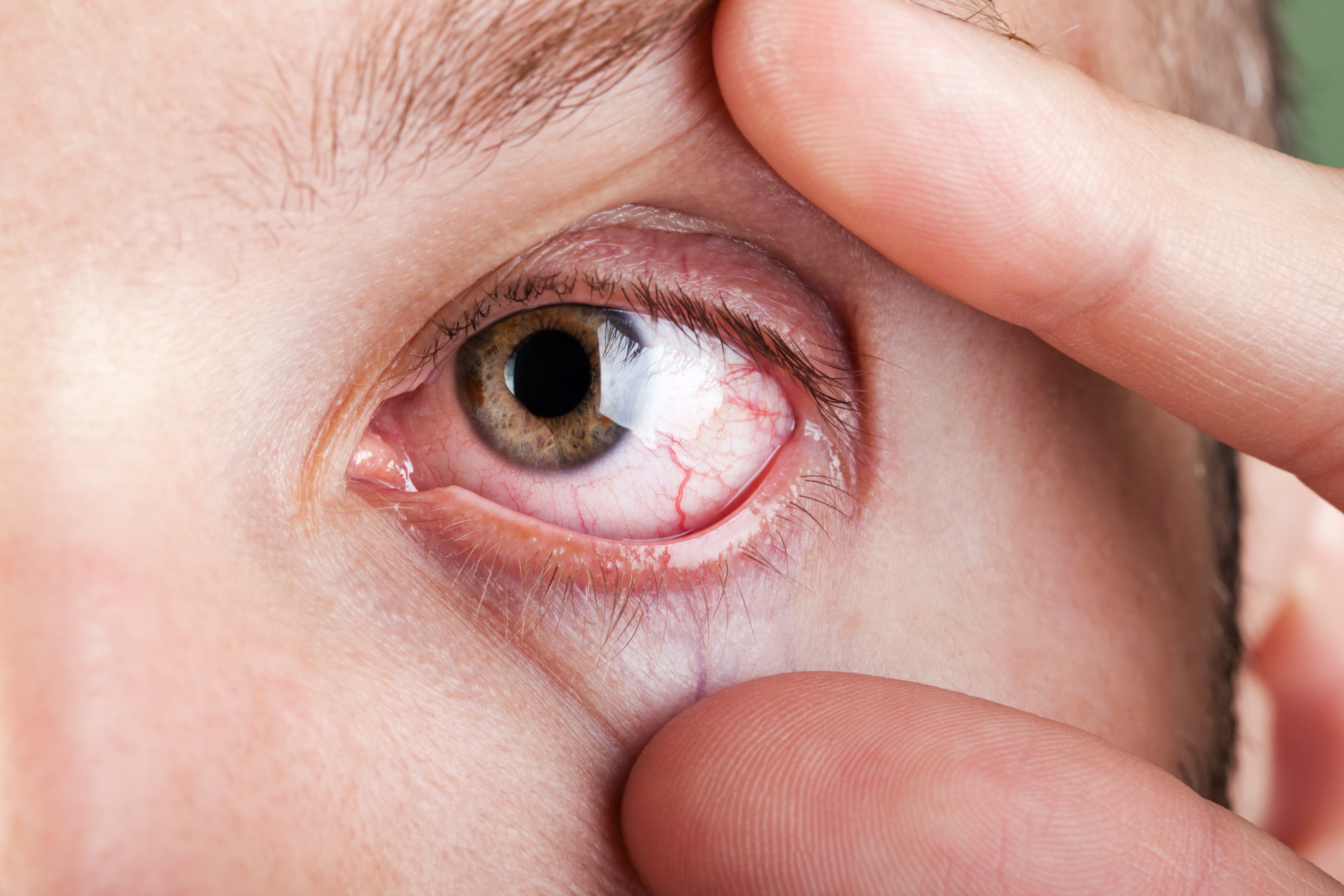
According to the American Academy of Ophthalmology, approximately 20 million people in the United States* (344 million people worldwide) have Dry Eye Disease, and that number is growing in both young and old adults, making it imperative that clinicians figure out how best to diagnose and treat it. According to the American Journal of Ophthalmology, as of July, 2017, a study reported an estimated 6 million people reported having experienced dry eye Disease symptoms but had never been diagnosed.
Diagnosing DED is a challenge because it’s a multifactorial disease, with many disparate causes. It has a highly variable symptom profile at different stages of the disease, and there’s often a discordance between signs and symptoms.
CLINICAL NEED FOR POINT-OF-CARE TESTING
– Reduces physician chair time
– Able to obtain rapid test results
– Patients prefer lab testing
– Improved patient care
– Treatment chosen specifically for patient test result
– Reimbursable through CMS and private insurance
– Very accurate, objective, differential diagnosis
– No out-of-pocket cost to patients
– New tests are being developed every day due to ongoing need for solutions
RENOWNED SCIENTIFIC MEDICAL ADVISORY BOARD
JOSEPH TAUBER, MD | CHAIRMAN
Dr. Tauber is the founder and CEO of Tauber Eye Center, a practice focused on corneal disease, uveitis and ocular immunology and complex corneal surgical procedures as well as Medical Director of Saving Sight, the US’ third largest eye bank.
Dr. Tauber has been centrally involved in virtually every significant dry eye development project during the past 25 years. He has served as a Principal Investigator in over 140 multicenter clinical trials including those that led to the approval of all four medications currently approved by the FDA for the treatment of dry eye – Restasis, Xiidra, Cequa and Eyesuvis. He has been avidly involved in research for nearly three decades, and a principal investigator in over 140 research studies across a broad range of eye conditions, including high-risk corneal transplantation, inflammation and allergic eye diseases, corneal infectious diseases and numerous ocular surface conditions.
Dr. Tauber received his doctorate from Harvard Medical School, residency training in internal medicine at Beth Israel Hospital and in ophthalmology at Tufts-New England Medical Center, and fellowship training in Ocular Immunology and in Corneal Diseases and Surgery at the Massachusetts Eye & Ear Infirmary, all in Boston, Massachusetts.
Dr. Tauber has also written eight book chapters and over 80 peer-reviewed articles in the fields of ocular surface and immunologic disease for prestigious medical journals as Ophthalmology, Investigative Ophthalmology and Visual Science, Journal of Cataract and Refractive Surgery and Cornea. He has been awarded the Heed Ophthalmic Foundation Fellowship Award and a National Eye Institute Individual NRSA Award.
DARRELL E. WHITE, MD
Dr. Darrell E. White is the founder and President of SkyVision Centers, among the first patient-centered eye care centers in the United States. SkyVision was launched with the treatment of Dry Eye Disease as its primary medical mission. Dr. White has been widely acknowledged as one of the leading voices in Dry Eye for more than 20 years. His Blog “The Dry Eye” is among the most read commentaries on both the science and business of treating Dry Eye Disease.
KELLY K. NICHOLS, OD., MPH., PhD
A founding member of the Ocular Surface Society of Optometry, Dr. Nichols currently serves as Dean of the School of Optometry at The University of Alabama at Birmingham. She is an acknowledged expert on DED and Ocular Surface Disease and has been extensively published. She earned her second B.S. and a Doctor of Optometry (O.D.) at UC Berkeley, and an M.P.H in biostatistics and a Ph.D. in Vision Science at Ohio State University.
LAURA M. PERIMAN, MD
Dr. Periman brings 30 years’ experience in medicine, the last 20 of which include her clinical practice specializing in ocular surface disease and dry eye disease (DED). She serves as Founder and Director of Dry Eye Services and Clinical Research of the Seattle-based Periman Eye Institute. Additionally, she has served as a principal investigator in ophthalmic clinical research primarily centered on ocular surface disease innovations including neural stimulation for treating DED, novel topical therapeutics as well as innovative procedures such as IPL, Radiofrequency and more
MICHAEL E. STERN, MS, PhD
Dr. Stern brings over 30 years of senior scientific, research, academic and executive level expertise with Dry Eye Disease and Ocular Surface Disease (OSD). Currently, he is a Principal and Chief Science Officer for immunEyze, a boutique contract research organization that performs preclinical and clinical research for OSD indications. Previously, he served for 26 years with Allergan where he rose to Principal Scientist and Vice-President Inflammation Research whose work included elucidating the pathophysiology of DED. He is extensively published in leading ocular journals.
*Source: USA General Population August 2018 General Census
